Pediatric Cardiac Assessment
Early detection of congenital heart disease (CHD) is crucial to save lives and improved outcomes. While prenatal screening programs often target high-risk pregnancies for detailed fetal cardiac assessments, it's important to recognize that the majority of CHD cases occur in the low-risk population (Hunter, 2014). To ensure the timely discovery and beneficial intervention for newborn congenital disorders, the US Advisory Committee on Heritable Disorders in Newborns and Children recommends that every newborn is evaluated for the Recommended Uniform Screening Panel (RUSP) of disorders, which currently includes 38 core disorders and 26 secondary disorders that can benefit from early detection. Ventricular Septal Defect is one of the 26 secondary disorders.
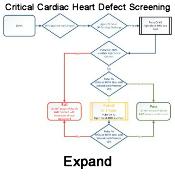 To reduce the risk of discharging a neonate with an undiagnosed hypoxic condition, the American Academy of Pediatrics has recommended newborn screening for Critical Congenital Heart Disease, outlined in their recent clinical report,Newborn Screening for Critical Congenital Heart Disease: A New Algorithm and Other Updated Recommendations: Clinical Report, published in January 2025. This updated algorithm utilizes pulse oximetry on the neonate's upper and lower limbs. While the primary goal is to detect CCHD, a positive screen indicates hypoxemia, it may also reveal other underlying hypoxic conditions such as sepsis, pneumonia, or persistent pulmonary hypertension of the newborn, prompting further urgent evaluation.
To reduce the risk of discharging a neonate with an undiagnosed hypoxic condition, the American Academy of Pediatrics has recommended newborn screening for Critical Congenital Heart Disease, outlined in their recent clinical report,Newborn Screening for Critical Congenital Heart Disease: A New Algorithm and Other Updated Recommendations: Clinical Report, published in January 2025. This updated algorithm utilizes pulse oximetry on the neonate's upper and lower limbs. While the primary goal is to detect CCHD, a positive screen indicates hypoxemia, it may also reveal other underlying hypoxic conditions such as sepsis, pneumonia, or persistent pulmonary hypertension of the newborn, prompting further urgent evaluation.
Current recommendations emphasize universal screening for critical congenital heart defects (CCHD) in newborns 24 hours after birth but before hospital discharge, typically in well-baby and intermediate care nurseries. Integrating this screening with the newborn hearing screen can optimize efficiency. Pulse oximetry, which measures the percentage of oxygen-saturated hemoglobin in the blood, is the standard method employed for this screening (CDC, 2022). The most recent guidelines recommend a passing oxygen saturation of ≥95% in both the pre-ductal (right hand) and post-ductal (either foot) measurements, performed while the infant is in room air (FiO2 of 21%) (Oster et al., 2025).
For infants who do not pass the initial screen, only one retest is now recommended (Oster et al., 2025).
It's important to note that the clinical presentation of a VSD can evolve in the weeks following birth. Often, a VSD may not be readily apparent until 4 to 8 weeks of age due to the initially high pulmonary vascular resistance (PVR) in the newborn. In the immediate postnatal period, the alveoli in the lungs are not fully developed, leading to elevated PVR. This high resistance limits the amount of blood shunted from the higher-pressure left ventricle to the lower-pressure right ventricle through the VSD.
The remainder of this page focuses on the infant cardiovascular assessment, which includes: a thorough review of the maternal, familial, antenatal, intrapartum, postnatal histories, and a physical examination to include; observation, physical inspection, palpation, auscultation, to detect potential congenital heart conditions.
Medical history
Maternal, perinatal, and infant history can provide important information that can help identify diagnostic clues that may indicate congenital heart defects or other cardiovascular issues.
- Familial factors
- Overall there is a 3 times increased risk for CHD when a first-degree relative has a CHD
- With each pregnancy, a parent with the defect has a 50% chance to have a child with the same heart defect, Males and females are equally affected (Stanford Children's Health n.d.).
- Maternal factors
- Seizure disorder and the need to take antiseizure medicines
- Taking lithium to treat depression
- Having phenylketonuria (PKU) and not staying on the special PKU diet during pregnancy
- Insulin-dependent diabetes, especially if blood sugar is not well-controlled
- Lupus A connective tissue disorder
- Rubella during her pregnancy has a very high chance of having a baby with birth defects, including CHD.
- A pregnancy from assisted reproductive technology, (Stanford Children's Health n.d.).
- Antenatal cardiac screening typically includes fetal echocardiograms and ultrasounds, which can identify structural abnormalities in the fetal heart. These scans may reveal various defects like ventricular septal defects, atrial septal defects, aortic atresia, and hypoplastic left heart syndrome.
- Initial post-delivery assessment record
- APGAR Score recorded at 1, 5, and 10 minutes after birth, evaluating heart rate, breathing, muscle tone, reflex irritability, and color.
- Record newborn’s weight and head circumference
- Record whether urine and meconium have been passed
Physical assessment
Approximately 3 out of every 1,000 babies are born with a critical congenital heart defect (CCHD). CCHD can be life threatening and requires intervention in infancy. Unfortunately, some infants with CCHD are still discharged from the nursery to home, where they can quickly decompensate.
Key Steps in the Infant Cardiovascular Examination:
- Inspection and Observation
- Assess the baby’s overall comfort, skin color, breathing patterns, and any signs of cyanosis or dysmorphic features.
- Understand that the newborn's cardiovascular system is dynamic and changes significantly in the first few hours, days, and weeks of life due to the transition from fetal to newborn circulation (American Academy of Pediatrics 2023).
- Theorell reports that it is common for the neonate to exhibit symptoms of an autonomic hypersympathetic state, including: tachycardia, tachypnea, transient rales, flaring, retractions, and grunting in the first 20 to 30 minutes post-partum. After this initial period of hyperactivity reaches a peak, it begins to diminish, the neonate enters a second period of parasympathetic dominance resulting in sleep.
- Baseline vital signs and blood pressure checks in all extremities are necessary.
- Physical examination should assess general color, temperature, heart sounds, and pulses.
- Signs of low cardiac output in infants include pale skin, tachycardia, and irritability.
- Interventions for low cardiac output may involve correcting heart rate and administering fluids.
- Precordial examination:
- Congenital heart defects are often associated with physical abnormalities of the hands, limbs, head and neck
Palpation
- Pulse Assessment: Check for simultaneous pulses at the brachial and femoral arteries to rule out conditions like coarctation of the aorta.
- Precordium and Liver Palpation: Feel for PMI localization, presence of thrills (a palpable vibration), and liver enlargement, which can indicate heart problems.
Auscultation
The goal of auscultation is to differentiate normal sounds from abnormal sounds. Challenges to auscultation in neonates include rapid heart rate, small chest, potential for transient murmurs. Auscultation is an important ongoing assessment tool because cardiovascular maturation is dynamic which can cause previously occult defects to be revealed as the neonate progresses through infancy.
- Key aspects of auscultation include:
- Normal Newborn Heart Sounds:
- First Heart Sound (S1) primarily represents systole and the closure of the atrioventricular valves (mitral and tricuspid). It is typically louder and shorter than S2 at the apex.
- Second Heart Sounds (S2) primarily represents diastole and the closure of the semilunar valves (aortic and pulmonic). It is typically louder and higher pitched than S1 at the base of the heart (upper sternal borders). May be physiologically split, especially with inspiration .
- Systematic Approach to Auscultation (Burns et al., 2020).
- Importance of a consistent and systematic approach to ensure all areas are assessed.
- Optimal positioning of the neonate for auscultation (supine, quiet state) (Theorell 2002).
- Using an appropriately sized stethoscope with both bell and diaphragm.
- Traditional Cardiac Listening Areas and Newborn Considerations:
- Aortic Area: Second intercostal space, right sternal border. Best for aortic valve sounds and aortic stenosis murmurs.
- Pulmonic Area: Second intercostal space, left sternal border. Best for pulmonic valve sounds and pulmonic stenosis murmurs.
- Tricuspid Area: Fourth or fifth intercostal space, left lower sternal border. Best for tricuspid valve sounds and murmurs.
- Mitral (Apical) Area: Fifth intercostal space, midclavicular line (may be slightly more lateral in newborns). Best for mitral valve sounds and murmurs.
- Left Upper Sternal Border: Important for murmurs of pulmonic stenosis, atrial septal defect (ASD), and patent ductus arteriosus (PDA).
- Right Upper Sternal Border: Important for murmurs of aortic stenosis and some other congenital defects.
- Axillae: Listen for radiation of mitral regurgitation and peripheral pulmonary stenosis.
- Back (Left Scapular Area): Listen for murmurs of coarctation of the aorta and radiation of peripheral pulmonary stenosis.
- Identifying and Characterizing Heart Murmurs
- Definition of a heart murmur: Turbulent blood flow creating audible vibrations.
- Key characteristics to document:
- Timing: Systolic, diastolic, or continuous.
- Intensity: Grade I (very faint) to VI (Levine Grading Scale).
- Pitch: High, medium, or low.
- Quality: Blowing, harsh, rumbling, musical, machinery-like.
- Location (Point of Maximal Intensity - PMI): Where the murmur is heard loudest.
- Radiation: Where else the murmur can be heard.
- Effect of Position Changes (Dynamic Auscultation): How the murmur changes with position (if applicable and feasible in a neonate).
- Common Newborn Murmurs and Associated Cardiac Defects
- Patent Ductus Arteriosus (PDA) (Rudolph & Hoffman, 2009; Hoffman, 2017):
- Description: Continuous "machinery-like" murmur, often loudest at the left upper sternal border and infraclavicular area.
- Note: May be softer or absent in premature infants.
- Aortic Stenosis (Park, 2014):
- Description: Systolic ejection (crescendo-decrescendo) murmur, best heard at the right upper sternal border, often radiating to the neck.
- Pulmonary Stenosis (Reference: Park, 2014):
- Description: Systolic ejection (crescendo-decrescendo) murmur, best heard at the left upper sternal border, radiating towards the left shoulder and back.
- Mitral Regurgitation (Allen et al., 2012):
- Description: Holosystolic (pansystolic), harsh murmur, radiating to the axilla. Less common as a primary congenital defect in newborns.
- Ventricular Septal Defect (VSD) (Wren et al., 1999):
- Description: Holosystolic (pansystolic) murmur, often loudest at the left lower sternal border.
- Note: Smaller VSDs typically have louder, harsher murmurs; larger VSDs may have softer murmurs.
- Peripheral Pulmonary Stenosis (Hoffman, 2017):
- Description: High-frequency systolic ejection murmur, often heard best in the axillae and back. Common and often transient in newborns.
- Coarctation of the Aorta (Burns et al., 2020):
- Description: Systolic ejection murmur heard best in the left interscapular area of the back. May also present with a continuous murmur if collateral flow is significant.
- When to Suspect a Pathological Murmur
- Red Flags:
- Loud murmurs (Grade III or greater).
- Diastolic murmurs (almost always pathological).
- Continuous murmurs (beyond the immediate newborn period).
- Murmurs associated with cyanosis, poor feeding, tachypnea, tachycardia, hepatomegaly, or other signs of cardiac compromise.
- Murmurs associated with abnormal pulses or blood pressure discrepancies.
- Important to correlate auscultation findings with the overall clinical picture.
- Need for further investigation (e.g., echocardiogram, consultation with a pediatric cardiologist) when a pathological murmur is suspected.
- Conclusion
- Reinforce the vital role of careful and systematic auscultation in the initial assessment of newborns.
- Emphasize the importance of ongoing learning and refinement of auscultation skills.
- Highlight the collaborative nature of care and the importance of communicating findings to the healthcare team.
Nursing considerations
As the infant's lungs mature and pulmonary circulation expands, the PVR naturally decreases. When the PVR falls below the systemic vascular resistance and the pressure generated by the left ventricle, a left-to-right shunt of blood across the VSD will occur, moving from the area of higher pressure to lower pressure.
If an infant or young child exhibits signs and symptoms of heart failure, a comprehensive cardiac assessment is warranted. Clinical findings suggestive of a VSD include:
- A characteristic heart murmur: Typically auscultated between 4 to 8 weeks of age, this is often described as a loud, harsh, holosystolic murmur.
- Best heard at the left lower sternal border (3rd or 4th intercostal space). The murmur often radiates widely across the precordium, and a palpable thrill may be present.
- Signs of right ventricular overload: The increased blood flow through the VSD into the right ventricle leads to increased workload and eventual right ventricular hypertrophy (RVH). This can be evidenced by:
- Radiographic findings: Chest X-rays may reveal cardiomegaly with right ventricular enlargement and increased pulmonary vascular markings due to increased pulmonary blood flow.
- Electrocardiogram (ECG) findings: The ECG may show signs of RVH, such as right axis deviation and increased right ventricular forces.
- Echocardiography (ECHO): This non-invasive ultrasound of the heart is a primary diagnostic tool for VSD. It can visualize the defect directly, determine its size and location, and assess the direction and magnitude of the shunt. ECHO can also demonstrate right ventricular and pulmonary artery dilatation.
- Cardiac Magnetic Resonance Imaging (MRI): While not always the first-line imaging modality for initial diagnosis, MRI can provide detailed anatomical and functional information about the VSD and its impact on the heart and pulmonary vasculature.
- Cardiac Catheterization: This invasive procedure involves inserting catheters into the heart chambers and great vessels. In the context of VSD, cardiac catheterization can:
- Measure oxygen saturation levels: A step-up in oxygen saturation in the right ventricle compared to the right atrium indicates left-to-right shunting.
- Measure pressures: Elevated pressures in the right ventricle and pulmonary artery are often observed due to the increased blood flow.
- Assess pulmonary vascular resistance: This helps determine the severity of the VSD and the potential for pulmonary hypertension.
It is important to remember that pulse oximetry screening does not detect all CCHDs, with a reported sensitivity ranging from 50% to 76% (Oster et al., 2025).
It is important to remember that the universal pulse oximetry screening does not detect all CCHDs, with a reported sensitivity ranging from 50% to 76% (Oster et al., 2025). Therefore, thorough clinical assessment remains crucial. Notably, the implementation of CCHD screening has been associated with decreased infant mortality and decreased emergency hospitalizations related to these conditions (Oster et al., 2025). Newborn screening is ideally performed at at least 24 hours of age or as late as possible before discharge, often coinciding with the newborn hearing screen (Oster et al., 2025).
Instant Feedback:
What is the primary rationale for delaying CCHD screening of the new born for at least 24 hours after birth.
In normal circulatory anatomy, the oxygen saturation sensor placed on the (R) arm indicates which section of the aortic arch.
References
American Academy of Pediatrics (2023). Newborn screening for critical congenital heart defect (CCHD). Retrieved May 11, 2025, from https://www.aap.org/en/patient-care/congenital-heart-defects/newborn-screening-for-critical-congenital-heart-defect-cchd/#:~:text=Newborn%20screening%20is%20a%20public,are%20included%20in%20newborn%20screening
Hunter, L. E., & Simpson, J. M. (2014). Prenatal screening for structural congenital heart disease. Nature reviews. Cardiology, 11(6), 323–334. https://doi.org/10.1038/nrcardio.2014.34
Oster, M. E., Pinto, N. M., Pramanik, A. K., Markowsky, A., Schwartz, B. N., Kemper, A. R., Hom, L. A., Martin, G. R., and the SECTION ON CARDIOLOGY AND CARDIAC SURGERY, SECTION ON HOSPITAL MEDICINE, & COMMITTEE ON FETUS AND NEWBORN (2025). Newborn Screening for Critical Congenital Heart Disease: A New Algorithm and Other Updated Recommendations: Clinical Report. Pediatrics, 155(1), e2024069667. https://doi.org/10.1542/peds.2024-069667
Romer, A. J., Johng, S., Hsia, J., Scott, S., Reddy, A., & Gardner, M. M. (2022). Cyanosis in a Newborn Immediately after Birth. NEJM evidence, 1(2), EVIDmr2100060.
Stanford Children's Health. (n.d.). Factors that may lead to a congenital heart defect (CHD). Retrieved May 13, 2025, from https://www.stanfordchildrens.org/en/topic/default?id=factors-that-may-lead-to-a-congenital-heart-defect-chd-90-P01788
Wu, L., Li, N., & Liu, Y. (2023). Association Between Maternal Factors and Risk of Congenital Heart Disease in Offspring: A Systematic Review and Meta-Analysis. Maternal and child health journal, 27(1), 29–48. https://doi.org/10.1007/s10995-022-03538-8
 To reduce the risk of discharging a neonate with an undiagnosed hypoxic condition, the American Academy of Pediatrics has recommended newborn screening for Critical Congenital Heart Disease, outlined in their recent clinical report,Newborn Screening for Critical Congenital Heart Disease: A New Algorithm and Other Updated Recommendations: Clinical Report, published in January 2025. This updated algorithm utilizes pulse oximetry on the neonate's upper and lower limbs. While the primary goal is to detect CCHD, a positive screen indicates hypoxemia, it may also reveal other underlying hypoxic conditions such as sepsis, pneumonia, or persistent pulmonary hypertension of the newborn, prompting further urgent evaluation.
To reduce the risk of discharging a neonate with an undiagnosed hypoxic condition, the American Academy of Pediatrics has recommended newborn screening for Critical Congenital Heart Disease, outlined in their recent clinical report,Newborn Screening for Critical Congenital Heart Disease: A New Algorithm and Other Updated Recommendations: Clinical Report, published in January 2025. This updated algorithm utilizes pulse oximetry on the neonate's upper and lower limbs. While the primary goal is to detect CCHD, a positive screen indicates hypoxemia, it may also reveal other underlying hypoxic conditions such as sepsis, pneumonia, or persistent pulmonary hypertension of the newborn, prompting further urgent evaluation.